If you've ever had heartburn, you know that the name is accurate. It feels like your chest is literally burning. But why is this the case? Heartburn is really just a result of your stomach acid having too much hydrochloric acid. Almost all liquids are either acids or bases to some degree. Whether a liquid is an acid or base depends on the type of ions in it.
"It is one of the more striking generalizations of biochemistry - which surprisingly is hardly ever mentioned in the biochemical textbooks - that the twenty amino acids and the four bases, are, with minor reservations, the same throughout Nature."
- Francis Crick

Definitions of Acids and Bases
Acids as substances that dissolve in water to produce H+ ions, whereas bases are defined as substances that dissolve in water to produce OH− ions. In fact, this is only one possible set of definitions. Although the general properties of acids and bases have been known for more than a thousand years, the definitions of acids and bases have changed dramatically as scientists have learned more about them.
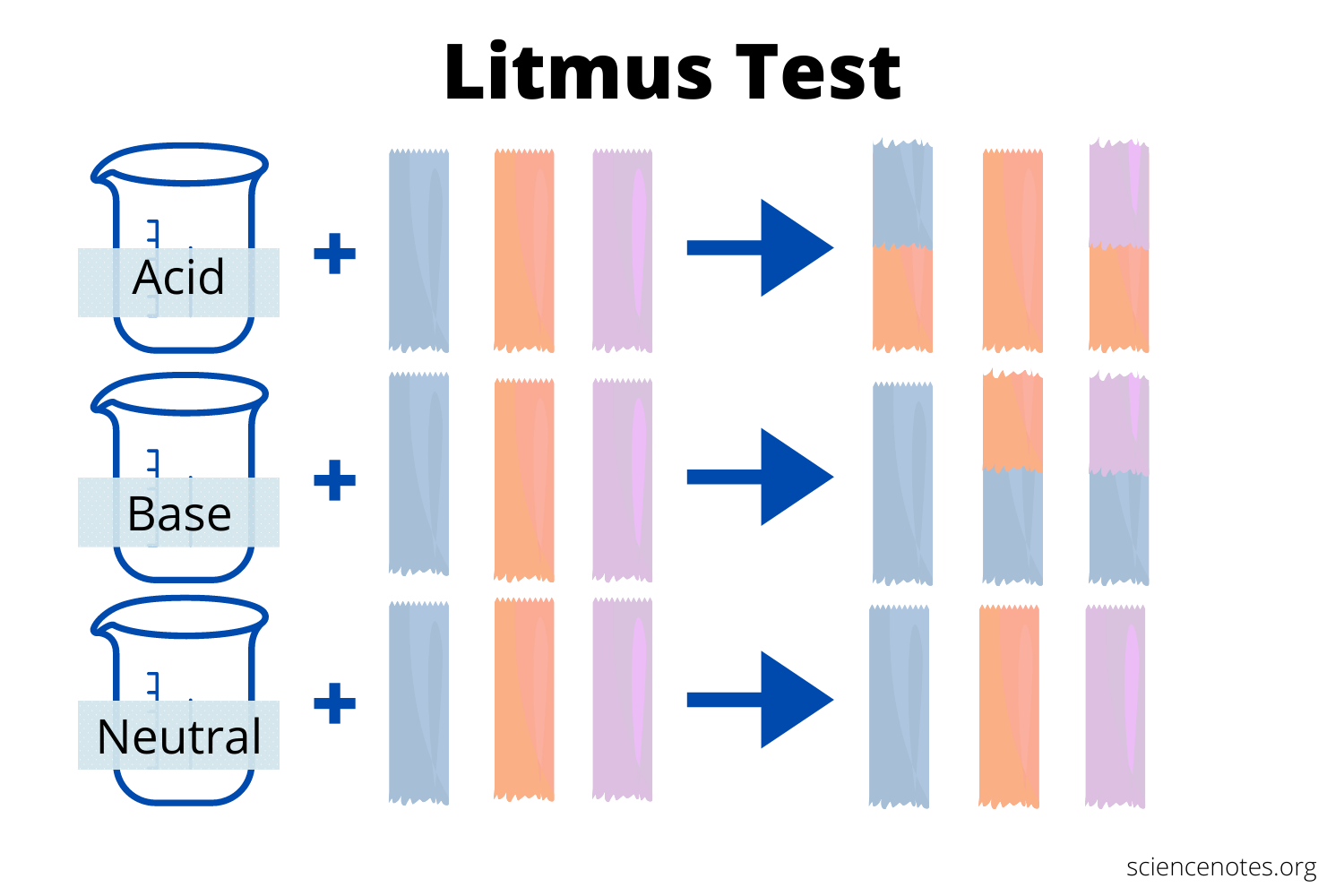
In ancient times, an acid was any substance that had a sour taste (e.g., vinegar or lemon juice), caused consistent colour changes in dyes derived from plants (e.g., turning blue litmus paper red), reacted with certain metals to produce hydrogen gas and a solution of a salt containing a metal cation, and dissolved carbonate salts such as limestone (CaCO3) with the evolution of carbon dioxide. In contrast, a base was any substance that had a bitter taste, felt slippery to the touch, and caused colour changes in plant dyes that differed diametrically from the changes caused by acids (e.g., turning red litmus paper blue). Although these definitions were useful, they were entirely descriptive.

Svante Arrhenius
The first person to define acids and bases in detail was the Swedish chemist Svante Arrhenius (1859–1927; Nobel Prize in Chemistry, 1903). According to the Arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce H+ ions (protons; Equation 4.6.1), and a base is a substance like sodium hydroxide that dissolves in water to produce hydroxide (OH−) ions (Equation 4.6.2)
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals.
According to Arrhenius, the characteristic properties of acids and bases are due exclusively to the presence of H+ and OH− ions, respectively, in solution. Although Arrhenius’s ideas were widely accepted, his definition of acids and bases had two major limitations:
- First, because acids and bases were defined in terms of ions obtained from water, the Arrhenius concept applied only to substances in an aqueous solution.
- Second, and more important, the Arrhenius definition predicted that only substances that dissolve in water produce H+
and OH−ions should exhibit the properties of acids and bases, respectively. For example, according to the Arrhenius definition, the reaction of ammonia (a base) with gaseous HCl (an acid) to give ammonium chloride (Equation 4.6.3) is not an acid-base reaction because it does not involve H+
and OH-

The periodic table of chemical elements, often called the periodic table, organizes all discovered chemical elements in rows (called periods) and columns.
Acid-Base Strength
The strength of acids and bases is based on how readily acids and bases perform their respective roles.
The strength of an acid depends on how well it dissociates and forms protons (H+ ions) in water. This means that a strong acid would more readily give up a proton than a weak acid, and a strong base more readily accepts a proton than a weak base.
Strong acids will completely dissociate and yield nearly 100% H+ ions in water, while weak acids will partially dissociate in H2O and not yield as many protons.
Strong acids donate a proton and become a weak conjugate base. Weak acids donate a proton and become strong conjugate bases. Similarly, the strength of a base depends on how well it dissociates in water, yielding hydroxide ions (OH-). Strong bases accept a proton and become weak conjugate acids. Weak bases accept a proton and become strong conjugate acids.
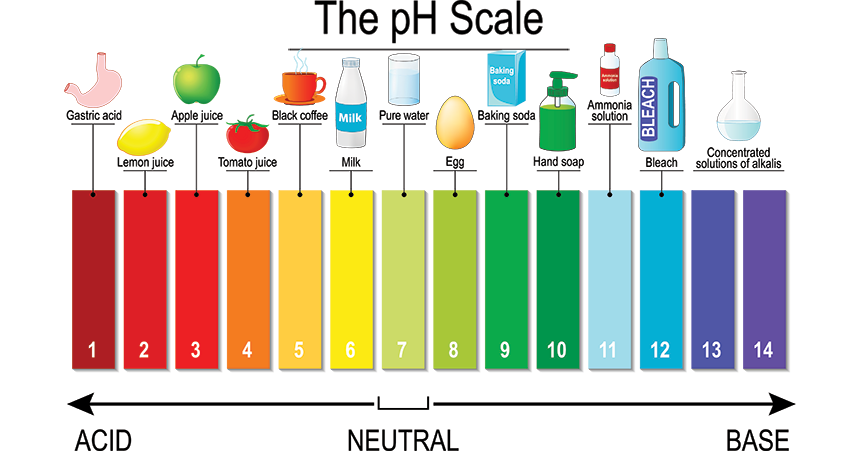
The pH Scale
A solution's pH is a measurement of the number of hydrogen ions (H+) and hydroxide ions (OH-) found in the solution. The pH scale is used in Chemistry to measure the tendency of molecules to be acidic or basic.
What Is pH?
The numbers of the pH scale come from a formula for the hydrogen ion (H+) concentration. The equation for pH is:
pH = -log[H+]
The way a logarithmic scale works, chemicals that donate more hydrogen ions (protons) have lower pH values. These are acids. Chemicals that accept hydrogen ions have higher pH values and are bases.
The atomic number of an element is the number of protons in the nucleus of an atom of that element.
Common Acids
Acids include chemicals with the word “acid” in their name. Examples include hydrochloric acid, acetic acid, and hyaluronic acid. Most fruits and vegetables are acidic. So is human skin and hair, which has a low pH to help protect the body from pathogens. Coffee, tea, wine, and milk are all acidic. Stomach acid is, of course, acidic.
Common Bases
Human blood is slightly alkaline. Baking soda (sodium bicarbonate) and washing soda (sodium carbonate) are bases. Cleaners are bases, including soap, detergent, bleach, ammonia, and drain cleaner.
Summarise with AI: