Chemistry is by far, one of the most interactive and fun subjects. Compared to other subjects where you study concepts through a textbook. There are lab lessons where you learn concepts by conducting various experiments. Scientists love it because they get to work in a field where you must constantly think creatively about overcoming problems. Overcoming problems and creating new ways to achieve this also builds your resilience and patience. It makes you develop your critical thinking skills and improves how you act in certain situations.
"Body concentrates order. It continuously self-repairs. Every five days you get a new stomach lining. You get a new liver every two months. Your skin replaces itself every six weeks. Every year, 98 per cent of the atoms of your body are replaced. This non-stop chemical replacement, metabolism, is a sure sign of life."
- Lynn Margulis
When you start to study the subject of chemistry it's usually a good idea, to begin with, atoms, and progress to elements, followed by molecules and compounds.

What Exactly are Atoms?
Okay, let's put this into words that actual humans can understand. I'm not a scientist but I find it so fascinating especially when it's explained to me in words that I know.
An atom is the basic building block of chemistry. It is the smallest unit into which matter can be divided without the release of electrically charged particles. An atom is a fundamental piece of matter. (Matter is anything that can be touched physically.) Everything in the universe (except energy) is made of matter, and, so, everything in the universe is made of atoms.
An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons.
About 99% of the human body is made up of atoms of hydrogen, carbon, nitrogen and oxygen. Suzanne Bell, an analytical chemist at West Virginia University, estimates that a 150-pound human body contains about 6.5 octillion atoms most of which were hydrogen, I can assure you that anything octillion is a number with so many zeros you've never seen it written down before.
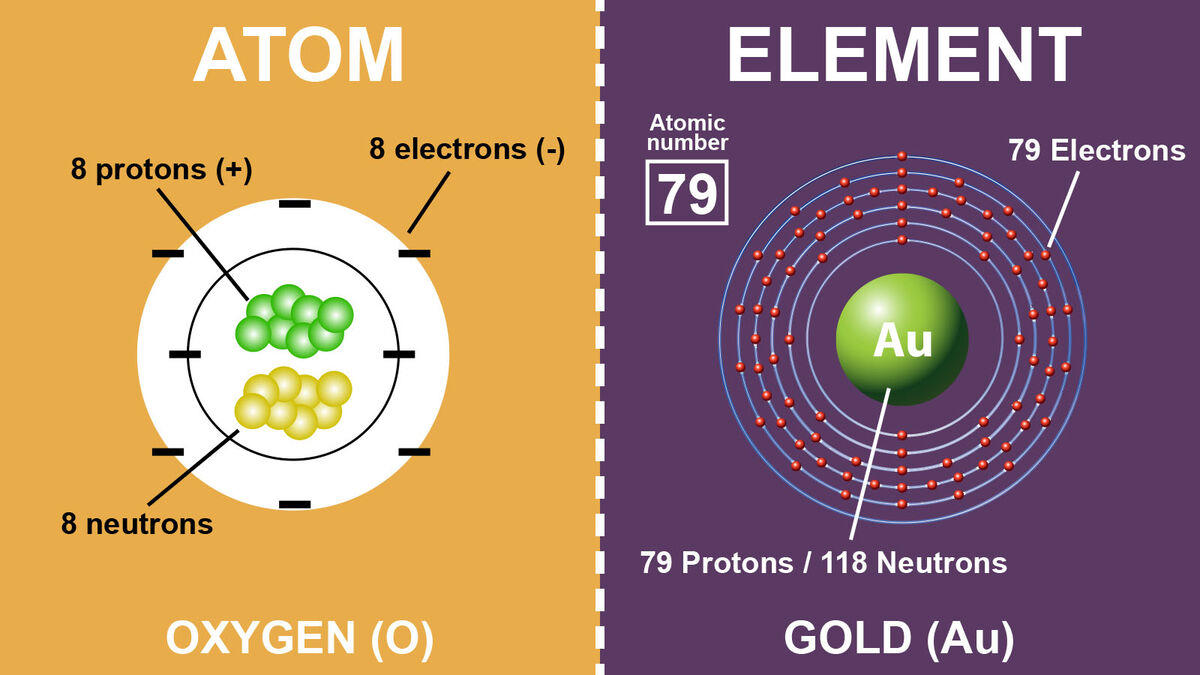
Elements
You will have noticed that when I mentioned the human body was made of atoms, I also mentioned elements. This is because all elements are made of atoms. An Element is a pure substance and is made of only one type of atom; it cannot be broken down into a simpler substance.
If you’ve ever seen a Periodic Table of Elements, then you probably have some idea of what elements are.
But to break it down into a simple definition, elements are all the different types of atoms we know exist on Earth. They are arranged by their atomic number on the Periodic Table of Elements.
Put simply what makes something an element is the fact that all the atoms have the same number of protons in the nucleus. Below is an example of the common elements mercury and copper:
- Mercury is an element with 80 protons in its nucleus. It has an atomic number of 80.
- Copper is made of atoms with 29 protons in the nucleus. Therefore, it has an atomic number of 29.
Chemical bonding is one of the most basic fundamentals of chemistry that explains other concepts such as molecules and reactions.
Molecules
With atoms and elements, all cleared up, it’s important to understand the difference between a molecule and an element.
Molecules are what you get when those atoms are combined. Unlike elements, molecules can be made from the same or different elements. The key to a molecule is that two or more atoms are bonded together. If they contain more than one atom, the atoms can be the same (an oxygen molecule has two oxygen atoms) or different (a water molecule has two hydrogen atoms and one oxygen atom). Biological molecules, such as proteins and DNA, can be made up of many thousands of atoms.
For example, water is a molecule made of hydrogen and oxygen. It’s actually made of two hydrogen atoms and one oxygen atom. You can also have molecules of a single atom bonded together like two oxygen atoms. Oxygen is found naturally as a molecule. Two oxygen atoms strongly bind together with a covalent double bond to form dioxygen or O2. Oxygen is normally found as a molecule. It is called dioxygen.
Acids and bases are an important part of the chemistry curriculum, and you also encounter acids and bases every day.
Molecules of Life
All life on Earth is built from four different types of molecules. These four types of molecules are often called the molecules of life.
The four molecules of life are proteins, carbohydrates, lipids and nucleic acids.
- Proteins are the first of the molecules of life and they are really the building blocks of life. Proteins are the most common molecules found in cells. If all the water is removed from a cell, proteins make up more than half of the remaining weight.
- Carbohydrates are an important source of energy. They also provide structural support for cells and help with communication between cells. A carbohydrate molecule is made of atoms of carbon, hydrogen and oxygen. They are found in the form of either sugar or many sugars linked together.
- Lipids are a highly variable group of molecules that include fats, oils, waxes and some steroids. These molecules are made mostly from chains of carbon and hydrogen called fatty acids. Fatty acids bond to a range of other types of atoms to form many different lipids.
- Nucleic Acids. There are two types of nucleic acids that are essential to all life. These are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
- DNA is a very well-known type of molecule that makes up the genetic material of a cell. DNA is responsible for carrying all the information an organism needs to survive, grow and reproduce.
- RNA is a lesser-known molecule but it also plays an important role in cells. RNA molecules are used to translate the information stored in DNA molecules and use the information to help build proteins. Without RNA, the information in DNA would be useless.
Summarise with AI: