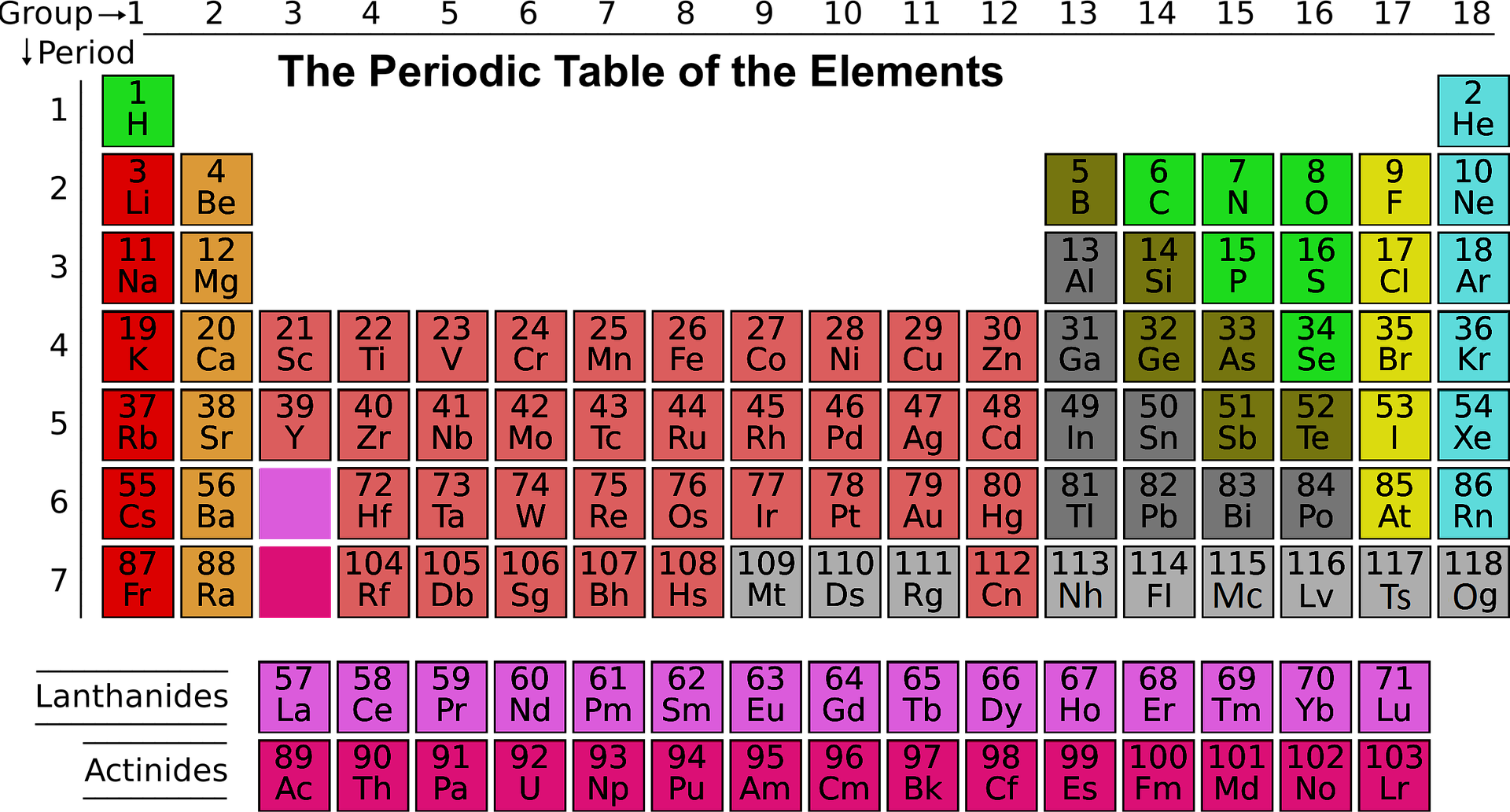
Students usually find the Periodic Table challenging to understand and memorise.
Yet for so many different careers, the Periodic Table is extremely helpful, for example, Chemists can synthesize novel molecules more easily once they have a better understanding of such interactions. Even better, it allows them to foretell the properties of newly formed compounds.
In this post, we are going to explore the Periodic Table in more detail and try some tricks to memorise the elements.


History of the Periodic Table
In February 1869 Dmitri Mendeleev, a Russian chemist and inventor, recorded on paper the symbols for the chemical elements, putting them in order according to their atomic weights and inventing the periodic table. He wrote down the sequence in such a way that they ended up grouped on the page according to known regularities or ‘periodicities’ of behaviour.
It was perhaps the greatest breakthrough in the history of chemistry, he was the first to create a table that classified elements by their atomic number, and it has been used ever since.
In 1913, Henry Moseley introduced the atomic number of elements through his X-ray study. He passed X-rays to the element and noted down the wavelength of elements, which he then converted into atomic numbers. After he assigned the atomic numbers to all elements, Moseley arranged them and constructed the modern periodic table, in the form of rows and columns.
It is called the periodic table because of the way the elements are arranged. The table is arranged into columns and rows. The vertical columns are called 'groups' and the horizontal rows are called 'periods'. Elements that show up in the same groups (columns) have similar properties.
Did you know that from left to right on the periodic table, the acid-base character of oxides and hydroxides goes from basic to acidic?
Facts About the Periodic Table
Now that we know when and how the modern periodic table came about it seems time to dive a little deeper.
How Many Elements on the Periodic Table?
At present, 118 elements are known to us. All these have different properties. Out of these 118, only 92 are naturally occurring the rest are synthesized in the labs. When you think about it, 118 elements, plus their symbol and atomic number, that's a lot of information to remember.
The Practical Uses of the Periodic Table
Think of the Periodic Table as a recipe book for scientists.
Basically, if you want to have the proper and complete understanding of the chemical elements, then it’s the periodic table which you will need to access since in this table all the chemical elements are arranged in a systematic manner just like you arrange all the spices on the shelf of your kitchen in order to prepare a portion of delicious food using them.
Or, you could think of it like the Formula and Tables ("Log Tables") Booklet, you get for Math exams. The periodic table is like a little encyclopedia of elements. The table tells you pretty much everything about all 118 elements, what each one is, its size, how it reacts, its physical state, etc.

How to Memorize the Periodic Table
Whether it's because of an assignment or simply because you want to know it, taking on learning the Periodic Table is no simple task, but you can do it!
The first step is getting a periodic table to study, and we all know that when it comes to studying repetition is key!
Here are tips that may help you memorize the table:
- Break the Periodic Table into Sections. You could memorize element groups (different colour groups), go one row at a time, or memorize in sets of 20 elements. It may be helpful to view an ordered list of the elements. Rather than attempting to memorize all of the elements at once, learn one group at a time, master that group, and then learn the next group until you know the whole table.
- Spread out the Memorization Process. You'll remember the table much better if you spread out the memorization process over multiple sessions instead of cramming the entire table at once. To truly commit the periodic table to memory, you need to access the part of your brain responsible for long-term memory. This involves repeated practice and exposure.
- Use Mnemonics for Memorizing Element Names. For a start, you can try a few examples like the ones below. It might inspire you to create your own.
- Alkali Metals - Highly Naive Kids Rub Cat’s Feet - Hi, Na, K, R, Cs, Fr
- Alkali Earth Metals Becky (&) Megan Can Send Back Reports - Be, Mg, Ca, Sr, Br, RaHalogens
- First Classic Brother In Atlantic - F, Cl, Br, I, At Noble Gases
- He Never Argued; Kevin Xeroxed Ronaldo - He, Ne, Ar, Kr, Xe, RnAnother
- Learn the Elements in a Song. You can learn a song someone else created or make up your own. There is a popular one called We Just Crammed the Table, which is set to a Billy Joel tune. This works well if you learn better by hearing information rather than seeing it on paper.
- Repetition is Key! Print multiple copies of the blank periodic table to practice filling in the symbols or names of the elements. It's easiest to learn the element symbols that go with the names, write in the symbols, and then add the names. Start small, with one or two rows or columns at a time.
The Periodic Table in a Nutshell
As we all know that chemistry is all about the chemical elements, which are used as the core properties of chemistry. The periodic table is like a little encyclopedia of elements. The table tells you pretty much everything about all 118 elements, what each one is, its size, how it reacts, its physical state, etc...
Summarise with AI: