"During the 20th century, we came to understand that the essence of all substances - their colour, texture, hardness and so forth - is set by their structure, on scales far smaller even than a microscope can see. Everything on Earth is made of atoms, which are, especially in living things, combined together in intricate molecular assemblages." -Martin Rees
As time goes on scientists make impressive discoveries in the field of biology, physics and chemistry. The hard work of a few motivated men and women benefits people all over the world.
Through scientific advancements, we have come to learn more about the reproductive system, the fact that the earth is round, the laws of gravity and that absolutely everything on earth is made up of atoms.
Understanding more about atomic structure can be done by studying physics in the last few years of secondary school. The Leaving Certificate Physics Syllabus, stems over two years and examines students on a diverse syllabus that surfaces invaluable learnings and a holistic understanding of the subject at both higher and ordinary levels.
Students will get to engage with the material associated with the topic of atomic structure through a series of problem-solving mathematics-based questions, definitions, long questions and a series of experiments.
This often provides students with a great medium for learning outside the norm of the typical classroom setting that you might have grown accustomed to throughout the leaving cert cycle across other subjects.
Superprof is here to guide potential students of physics and offer information on what to expect from the Leaving Cert Physics Syllabus. We will now focus on the fourth topic of atomic structure and what is learnt in this section.
In this article, I will delve into the material associated with Atomic Structure in both ordinary and higher level physics. I will also provide you with a summary of its assessment and the opportunities of learning more about the topic across the island of Ireland.
Do you need a physics and maths tutor to get a better grasp of this topic?
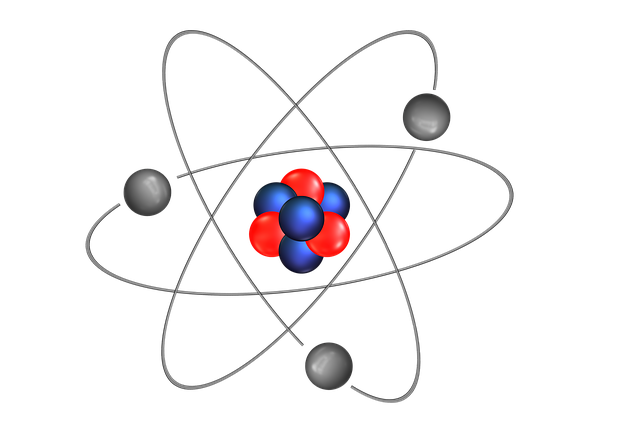

What exactly are Atoms and Ions?
Atoms are extremely small in size, in fact, they are actually microscopic and approximately 100,000,000 of them take up a space measuring one centimetre. They can exist on their own or together as molecules.
An atom has a nucleus containing protons and neutrons with smaller electrons that circle around the nucleus.
Each particle has its own mass and its own charge:
| Charge | Mass | |
|---|---|---|
| Electron | +1 | 1 |
| Neutron | 0 | 1 |
| Proton | -1 | Almost 0 or (1/2,000) |
Protons and neutrons are the heaviest particles in an atom and the total number of them in an element is known as the mass number and the number of protons by themselves is called the atomic number.
There are also electrons that are negatively charged. they are closely bound and unlike neutrons and protons, move freely around the atom.
The mass number and atomic number and both essential pieces of information about an atom. Atoms can be represented using the following symbol:
In this symbol A is the mass number, Z is the atomic number and X is the symbol or element. The numbers can be examined on the periodic table of elements which shows how many particles are in the nucleus and how many protons each element has.
Isotopes
In this section, students will also discover what exactly isotopes are. They can be defined this way, they are the forms of an element that have the same number of protons but different numbers of neutrons. For example, chlorine has two isotopes and carbon has three.
Ions
Atoms are normally neutral and have the same number of protons in the nucleus as they have electrons spinning around. Nevertheless, there are always exceptions. These exceptions can possibly occur when there is a loss or gain of electrons after a collision. When this happens, little particles are created and are called ions.
Ions can either be positively or negatively charged. They are positively charged if the atom loses one or more electrons and they are negatively charged if the atom gains one or more electrons.
Radioactive Decay
If nuclei have an incorrect number of neutrons they can quickly fall apart. This is also called radioactive decay.
Attaining Stable Nuclei
The nucleus of an atom can only be stable if it has a certain amount of neutrons for the number of protons it has. Elements that have very few protons and that can be found near the top of the periodic table are stable if they have the same number of neutrons and protons.
Nevertheless, it is important to note that as the number of protons increases, more neutrons are needed to keep the nucleus stable and troubles at bay!
A nucleus that has too few or too many neutrons does exist in nature but will decay and eventually emit radiation.
Ask your physics and maths tutor who pioneered the concept of nuclear decay!

Learn more about Atomic Structure at University!
The physics course that is on offer at UCD takes on about 420 students each year, so you will likely have peers that are looking to become teachers too! After all, you can always make money tutoring the subject outside of the lecture hall! The physics course is four years long and touches on just about everything that you would need to know if wanted to teach the subject.
Let’s take a quick look at the general physics that the college has on offer to students. First of all, you’ll have access to some of the best facilities that the country has to offer and experienced lecturers that will teach you just as much about teaching as physics. You’re bound to pick up a number of tricks in your lectures!
Studying a physics degree often presents students with a comprehensive knowledge of the core elements of the subject, allowing you to go above and beyond the leaving cert curriculum. The course includes maths for physical sciences, linear mathematics, calculus, differential equations as well as some abstract algebra.
Joining the colleges’ physics societies can be a great way to meet students who have shared interests and other recreational clubs. This is a great opportunity to socialise and make great friends on campus where there is no shortage of student life on the vibrant campus of UCD. This university should be involved in any conversation regarding studying physics in Ireland.
Bear in mind that candidates will also have to achieve at least a Grade H4 in Leaving Certificate physics if they want a place on the course, but there are plenty of options out there if you need to find an alternative route to becoming a teacher.
There are also countless options for college courses that you can take at university if you are pursuing a career in teaching physics. DCU is certainly one of the top choices that you should be considering if you are serious about becoming a physics teacher and securing full-time or part-time employment teaching the subject.
Studying Physics after the leaving cert surfaces an invaluable opportunity for any students looking to advance their competencies in physics and create some exciting career prospects in teaching for themselves thereafter. The work placements are extremely well perceived by students and often result in full-time employment after the candidate graduates with the degree.
By attaining a degree in physics from a reputable college such as Limerick university, you would inherently be putting yourself above the competition. This physics degree is a great way to ease your transition into a career in teaching after you graduate from the course.
Note: You can find an online Physics tutor on Superprof and get on track with your study.

Sample Exam Questions and Assessment on Atomic Structure
A key component of exam success is knowing what is expected of you.
For your physics exam, you are expected to recognise aspects of the course such as atomic structure, solve complex maths equations and define various terms at a level appropriate for your grade. Those aspects are obvious but others aren’t, like showing your workings, and how you present your work.
There are seven types of questions that can be encountered on an assessment for the topic of Electricity while studying the Leaving Certificate Physics Syllabus. The following are examples of those questions:
- Multiple choice questions: probably the easiest since all you have to do is tick a box. However, be warned that show of the answers may be similar and this is designed to trick you.
- One or two mark questions: they usually start with command words such as describe or explain. These can either be worth one or two marks and you have to describe a scientific topic using short sentences.
- Three or four mark questions: longer answers are required and valid points need to be explained in a logical manner.
- Maths questions: these questions may include graphs and tables and it is essential to show all of your work in order to get full marks.
- Practical questions: when studying Leaving Cert Physics you will need to complete eight practical activities and these assessment questions are based on those. Use all the information provided to write down the correct answer.
- Six mark questions: these are probably the most difficult and need to be answered logically. Planning and carefully reading the questions is essential for success.
- Equations: some of these questions will require you to recall and apply equations you learnt during class time.
Taking charge of your exam in this manner will ensure confidence come exam time, allowing you to perform at your best.
Studying the different sections of the atomic structure topic can prove to be very interesting and diverse. A thorough explanation of an atom, radioactive decay or uses of radiation is all covered in the Leaving Certificate Physics Syllabus and can be great fun for science enthusiasts!
Atomic structure is just one of the eight topics covered in the Leaving Certificate Physics Syllabus, the other seven include energy, electricity, particle model of matter, forces, waves, magnetism and electromagnetism and space physics.
Summarise with AI: